Science 2019; 363, 875–880


A pharmacological master key mechanism that unlocks the selectivity filter gate in K+ channels
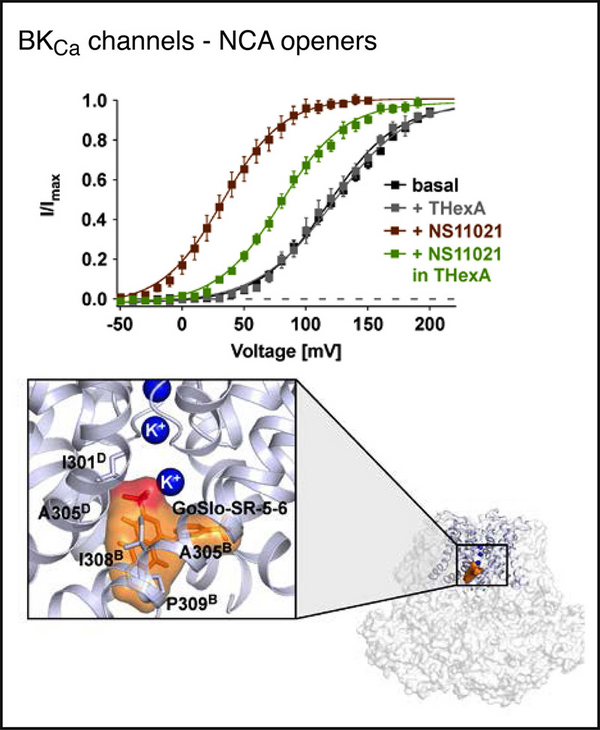
Potassium channels have been evolutionarily tuned for activation by diverse biological stimuli, and pharmacological activation is thought to target these specific gating mechanisms. Here we report a class of negatively charged activators (NCAs) that bypass the specific mechanisms but act as master keys to open K+ channels gated at their selectivity filter (SF), including many two-pore domain (K2P) channels, voltage-gated hERG (human-ether-a-go-go related gene) channels and calcium (Ca2+)-activated BK-type channels. Functional analysis, X-ray crystallography and molecular dynamics simulations revealed that the NCAs bind to similar sites below the SF, increase pore and SF K+ occupancy and open the filter gate. These results uncover an unrecognized poly-pharmacology among K+ channel activators and highlight a filter gating machinery that is conserved across different families of K+ channels with implications for rational drug design.



