Molecules. 2019;24(10)


Design of a human rhinovirus-14 3C protease-inducible caspase-3
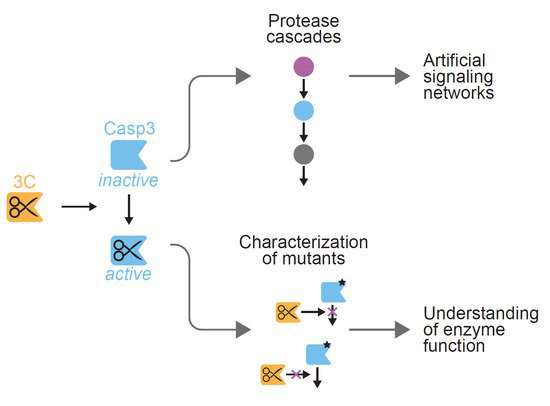
Here, we describe design strategies for the generation of a human rhinovirus-14 (HRV14) 3C protease-inducible caspase-3 (CASP3). We use our engineered CASP3 to characterize the effect of aspartate mutations on enzymatic activity. Besides the identification of mutations that render the enzyme inactive, we find the CASP3-D192E mutant (aspartate-to-glutamate exchange at position 192) to be inaccessible for 3C protease-mediated cleavage.