In our #NextGen Signalling Scientists series, we spotlight Early Career Researchers who are shaping the future of signalling research at CIBSS. This feature highlights molecular biologist Christoph Reiter, MD candidate at the Institute of Biochemistry and Molecular Biology at the Faculty of Medicine, University of Freiburg in the group of Prof. Dr. Claudine Kraft.
Christoph Reiter

“It is a fantastic experience to not only consume knowledge, but to contribute to it, even if it is only a small puzzle piece in the final picture.”
If your research in biological signalling studies were an object, image, or even a metaphor, what would it be?
If I were to describe my research through an image, it would be a recycling plant with a manager overseeing the process. The autophagy pathway functions as the cell’s recycling and waste management system. It maintains balance by breaking down and reusing damaged components. My work focuses on factors involved in a specialized form of autophagy called mitophagy. This pathway is responsible for degrading and recycling defective parts of mitochondria, the cell’s power plants.
We identified factors that act as manager of this cellular recycling plant. But they do not only coordinate the dismantling and recycling processes (mitophagy), they also recognize when the damage is beyond repair. If this is the case, apoptosis is triggered – a form of cell death that can be compared to a controlled shut-down of the cell to protect the system as a whole. Through this balancing act, a cell decides wisely between repair and reuse, or else demise.
Research is full of surprises, setbacks, and breakthroughs: What moment in your work, which discovery, challenge, or unexpected twist has been especially surprising or shaped your perspective? And what keeps you motivated to explore biological signalling further?
Although I study medicine and my path is more likely to lead me into the clinics and not necessarily directly into a research lab, I wanted to experience what it’s like to stand at the edge of discovery. The feeling of being right where new discoveries are made still motivates me. It is a fantastic experience to not only consume knowledge, but to contribute to it, even if it is only a small puzzle piece in the final picture.
One of the most rewarding moments came during one of my experiments, where I used a Phostag-gel. We use this method to visualize the phosphorylation of proteins, a process in which small phosphate-groups are attached to them. The first attempts didn’t work and I couldn’t see any phosphorylation. But I remained motivated, optimized my experimental set up and finally a clear phospho-shift appeared. It was an incredibly satisfying moment that made all my previous effort worthwhile.
Another highlight was attending the annual conference of the Nordic Autophagy Society, where I had the opportunity to talk to leading experts in the field. To experience their genuine curiosity about our work, and at the same time learn so much from the research of others was deeply inspiring.
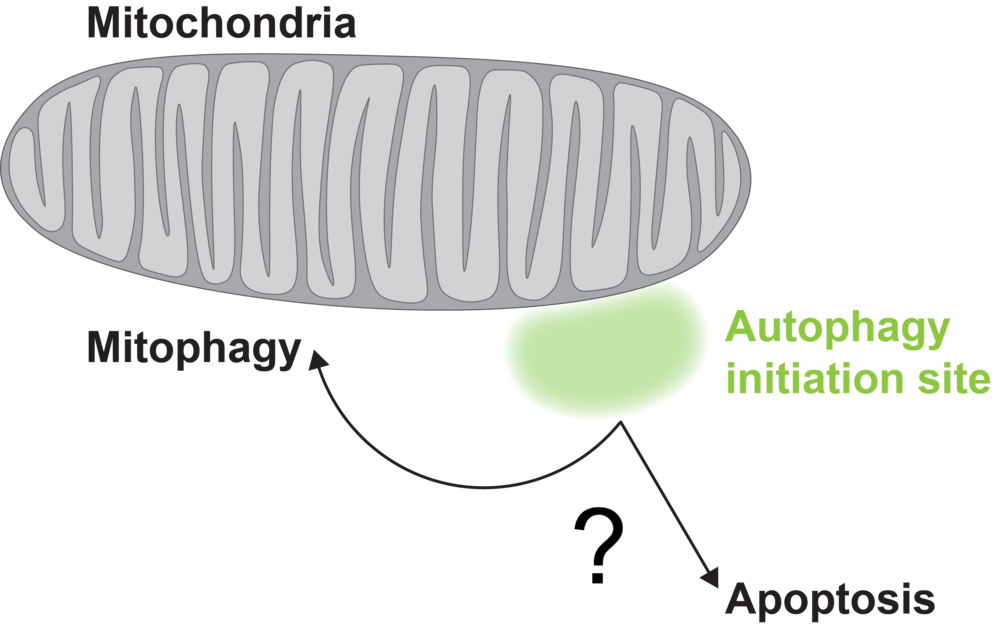
Mitophagy is responsible for degrading and recycling defective parts of mitochondria. Early on, several “manager” factors accumulate at the autophagy initation site to start the recycling process. They help deciding whether mitochondria can be recycled through mitophagy or should be safely removed through apoptosis, maintaining the cell’s balance between renewal and controlled shutdown.
Graphic: Christoph Reiter
If you could fast-forward, what major milestone or discovery within this field would you most hope to see? Why is this significant for the broader research landscape?
In the next few years, I hope to see more research exploring how mitophagy and apoptosis work together during the initiation of mitophagy. Another important aspect is how only parts of the mitochdrial network are selected for degradation, as not the entire network within a cell gets degraded. These connections are still not fully understood, and uncovering the signalling mechanisms that coordinate these pathways would be an exciting step forward.
Looking further ahead, it would be fascinating to understand how the communication between mitophagy and apoptosis affects human health. In the long run, I hope this knowledge can help us develop therapies that target and adjust this cellular communication. Such approaches could offer new ways to restore balance in cells affected by disease.



