In our series #NextGen Signalling Scientists, we spotlight Early Career Researchers shaping the future of signalling science at CIBSS. This feature highlights molecular immunologist Dr. Nadine Wössner, a researcher at the Department of Synthetic Immunology, at the Faculty of Biology, University of Freiburg, in the group of Prof. Dr. Susana Minguet.
Dr. Nadine Wössner

“What excites me most about my research is the opportunity to combine basic science with clinically relevant applications that may one day contribute to real-world solutions.”
If your research in biological signalling studies were an object, image, or even a metaphor, what would it be?
If my research were an object, it would be a light switch dimmer. A dimmer doesn’t just switch lights on or off – it adjusts the brightness to exactly what’s needed. In the same way, my work focuses on how T cells, a key part of our immune system, fine-tune their activation signals to respond to threats like infections or cancer cells.
Specifically, I study CD3ɛ, a subunit of the T cell receptor complex, which is a protein structure on the surface of T cells that allows these cells to recognise specific antigens (threats) and transmit activation signals into the cell. Here, CD3ε acts as a master regulator of T cell signalling. This subunit contains four key motifs that recruit both activating and inhibitory proteins, helping the immune system strike the perfect balance.
If this "dimmer" is too low, T cells can’t fight infections or cancer effectively. If it’s too high, it can lead to autoimmune diseases. My goal is to figure out exactly how this system works, so that we can design smarter therapies. This includes engineering more effective CAR T cells for cancer immunotherapy and developing precise inhibitors for treating autoimmune diseases. Ultimately, it’s about keeping the immune system “bright” enough to combat diseases but “dim” enough to protect our healthy tissue. ***
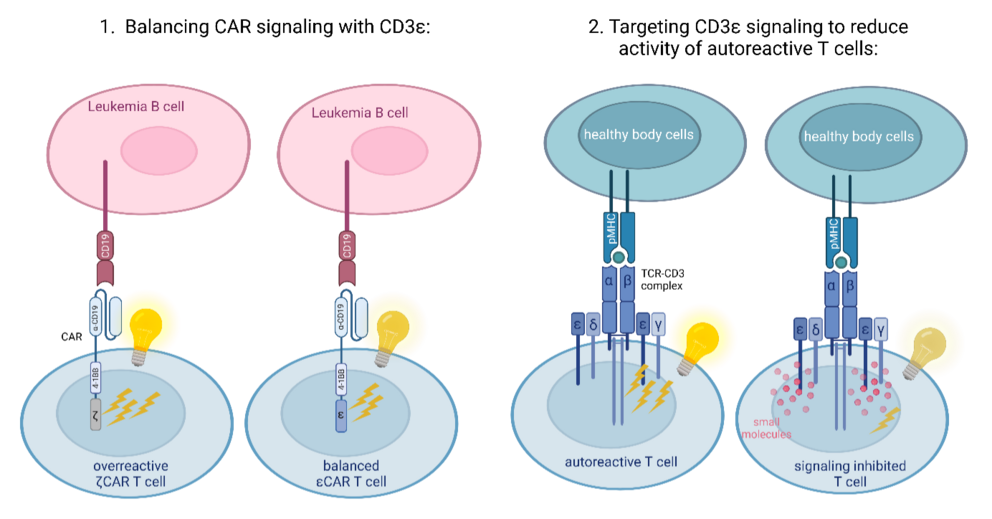
Targeting CD3ε Signalling for Safer CAR T Cell Therapy and Autoimmune Disease Treatment (Graphic: Nadine Wössner / University of Freiburg, created with Biorender).
Research is full of surprises, setbacks, and breakthroughs: What moment in your work, which discovery, challenge, or unexpected twist has been especially surprising or shaped your perspective? And what keeps you motivated to explore biological signalling further?
What drives me in research? Discovering potential! One of the most rewarding experiences in my work came from stepping into the world of drug development – a field that was completely new to me at the time. My goal was to identify small molecules that could modulate T cell activity by targeting the signalling pathways controlled by the CD3ɛ subunit. The idea was to “dim” overactive T cells, while allowing the rest of the immune system to function normally. It sounded straight forward, but the process was anything but.
The project required endless rounds of trial and error. Early experiments didn’t yield promising hits, and I spent months troubleshooting screening protocols and adjusting experimental conditions. There were moments of frustration when progress felt painfully slow. But with time, things began to fall into place. I refined the approach, optimised key steps, and eventually, we identified a new class of T cell modulators with the potential for broad applications in autoimmune diseases and immunotherapy.
This experience taught me the importance of patience, adaptability, and the value of creative problem-solving. What drives me is the thought of how these small molecules could one day make a real difference for patients – offering a potential alternative to the broad immunosuppressive therapies that often come with significant side effects.
Looking Forward: What’s next in your research journey? If you could fast-forward to 2025 and beyond, what milestone or discovery would you most hope to achieve?
Looking ahead, I’m excited about the future of CAR T cell therapies (genetically engineered T cells designed to target and destroy cancer cells) and their potential to become safer and more effective through precision engineering. By 2025, I hope to see CAR T cells with the CD3ɛ subunit in clinical trials, showing enhanced efficacy against tumours with fewer side effects compared to the traditional ζ-based CARs used today.
I also aim to advance the development of a next generation of small molecules that target CD3ɛ signalling. These innovative T cell modulators could provide new treatment options for a variety of T cell-driven autoimmune diseases, reducing the reliance on broad immune suppression therapies.
What excites me most about my research journey is the opportunity to combine basic science with clinically relevant applications that may one day contribute to real-world solutions.
*** We are exploring the signalling of the T cell receptor (TCR) subunit CD3ε to develop methods for precise control of T cell activation. This research has two important applications.
1. It offers a way to improve CAR T cell therapy, a treatment where T cells are engineered to recognize and destroy cancer cells. Traditional CARs typically use the ζ (zeta) subunit of the TCR, which can lead to excessive activation of the CAR T cells and cause severe side effects in patients. By incorporating the CD3ε subunit instead, we’ve observed more balanced signalling1–3. This adjustment not only improves the anti-tumor effectiveness and persistence of CAR T cells but also reduces the release of inflammatory cytokines, making the therapy potentially safer for patients.
2. We have developed small molecules that target the signalling activity of the CD3ε subunit. These molecules have the potential to become a treatment for autoimmune diseases, where T cells mistakenly attack healthy tissues. By selectively reducing T cell activation, these small molecules could minimise harmful autoimmune responses while preserving the overall function of the immune system.
- Hartl, F. A. et al. Noncanonical binding of Lck to CD3ε promotes TCR signaling and CAR function. Nat. Immunol. 21, 902–913 (2020). https://doi.org/10.1038/s41590-020-0732-3
- Velasco Cárdenas, R. M. H. et al. Harnessing CD3 diversity to optimise CAR T cells. Nat. Immunol (2023). doi:10.1038/s41590-023-01658-z
- Woessner, N. M. et al. Phospho-mimetic CD3 e variants prevent TCR and CAR signaling. Front Immunol 15, 1–14 (2024). https://doi.org/10.3389/fimmu.2024.1392933



