

PP2A, a key signalling hub throughout evolution
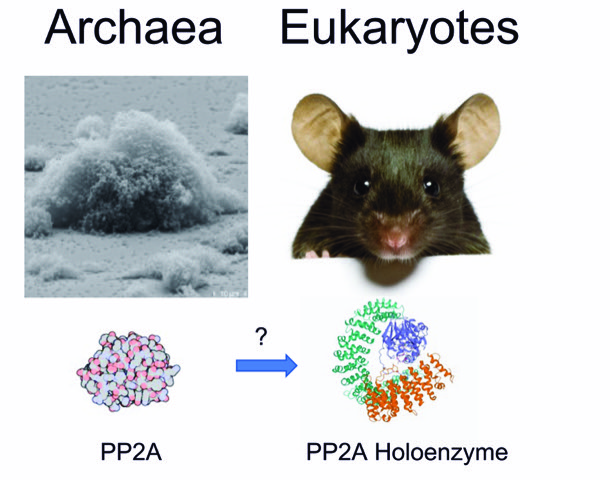
In this project, we will investigate evolutionary scales of cellular signalling by studying the serine/threonine-specific phosphatase PP2A. In mammalian cells, PP2A consists of a trimeric holoenzyme and is responsible for the dephosphorylation of thousands of phosphorylation sites. The catalytic subunit is evolutionary conserved. In the crenarchaeon Sulfolobus acidocaldarius PP2A is the only serine/threonine-specific phosphatase, however, we have detected more than 400 phosphorylation sites on serine and threonine, which could be potential substrates of this archaeal PP2A. We will study in a comparative manner how PP2A is regulated in different species, as well as if and which interaction partners and substrates are conserved, to reveal whether central signalling events exist over evolutionary scales.



