

Spatiotemporal signal integration in control of the neural stem cell niche and progenitor plasticity
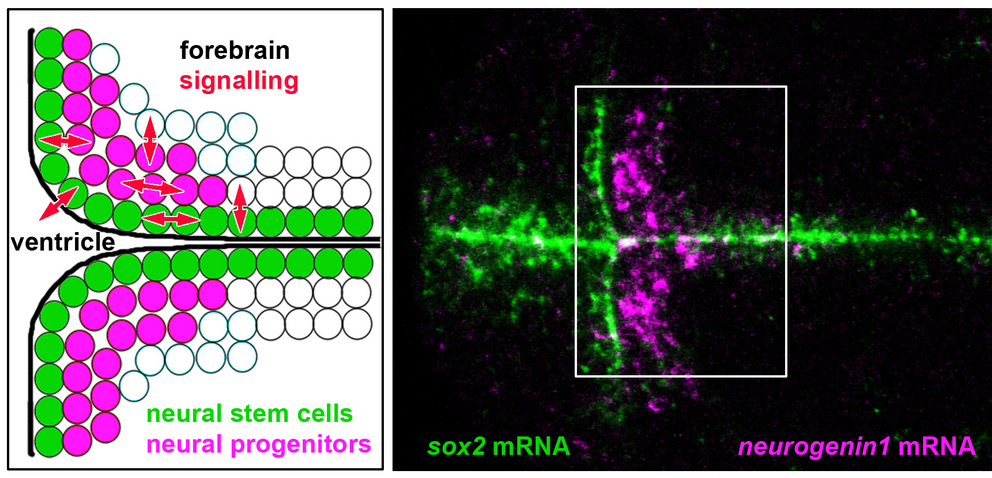
The brain is limited in its regeneration capacity after loss of neurons due to injury, stroke or degeneration. While neurogenesis in the embryo or in vitro from stem cell cultures is well understood, control of regeneration from endogenous neural stem cells in vivo is currently not possible. This project aims at an integrated understanding of temporal and spatial control of signalling molecules and transcription factors to elucidate dynamics and control of neural stem cell derived neurogenesis in vivo. We focus our efforts at early larval stages when neural stem cells niches originate from embryonic proliferation zones in the forebrain. We use the zebrafish model system, which is characterized by robust proliferation and easy experimental accessibility of larval neural proliferation zones and stem cell niches. We investigate the effects of Delta-Notch signalling-dependent and -independent HES/HER transcription factors on neural stem and progenitor cells in the context of regional Shh and WNT signalling. The mechanisms that establish the neural stem cell niche should provide unprecedented insights into regulation of the specific cell populations in the neural stem cells niche. The results may stimulate future approaches to compensate loss of neurons in the diseased, injured or aging brain.



