PD. Dr. Klaus-Peter Knobeloch (CIBSS-AI), Institute of Neuropathology, University of Freiburg, Medical Faculty
Prof. Dr. Wolfgang Schamel (CIBSS-PI), Institute of Biology III, Faculty of Biology, University of Freiburg
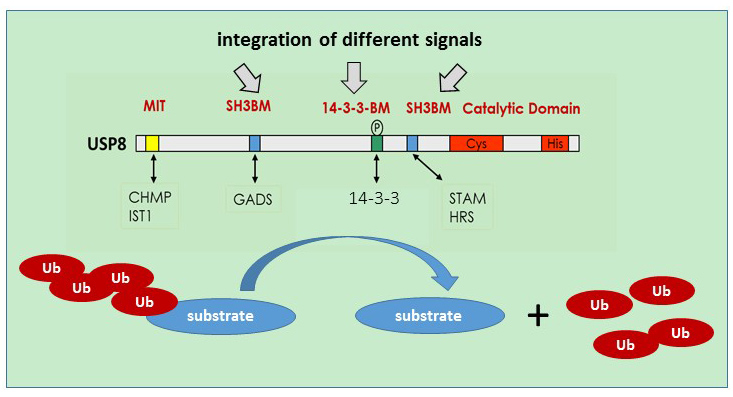
Ubiquitination plays a major role in the control of protein stability and signaling and is counteracted by deubiquitinating enzymes. USP8 is an ubiquitin-specific protease characterized by a multi-domain structure allowing the integration and regulation of catalytic activity and cell specific functions via specific protein-protein interaction modules and regulatory elements. We analyse how characteristic domains of USP8 integrate signalling and translate into cell-specific activities. Furthermore, we will define which upstream signals are in place and how this affects USP8 controlled substrate ubiquitination. Similar to our previous work, we combine in vitro approaches with knock-in mouse models harboring mutations in distinct domains of USP8 to evaluate USP8 molecular mechanisms and physiological relevance. A particular focus will be lymphocyte function and immunity.