A team of researchers at the Cluster of Excellence CIBSS at the University of Freiburg and at the ZMBH in Heidelberg has identified a pioneering signalling pathway that is responsible for the precise coordination of import processes in mitochondria. A study led by Prof. Dr. Chris Meisinger and Prof. Dr. Nora Vögtle was able to show how DYRK1A, a key kinase in the cytosol, synchronises the different import pathways in mitochondria and adapts them to the respective metabolic conditions. This discovery opens up new perspectives for the understanding of mitochondrial diseases, including those associated with genetic alterations such as Down syndrome. The study was recently published in Nature Communications (2024).


New insights into mitochondrial signalling integration
CIBSS researchers identify protein kinase DYRK1A as a central regulator of import pathways in mitochondria
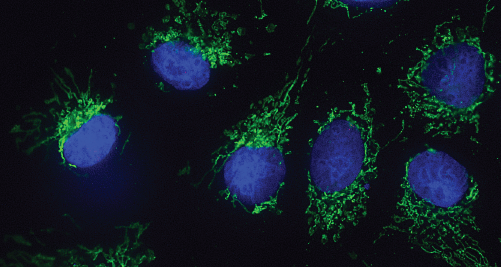
Fluorescence microscope image of mitochondria of human cells (light green), the cell nucleus is coloured blue. Image: Working group of Claudine Kraft.
The role of signalling integration at the mitochondrial “entry gate” for cellular energy metabolism
Mitochondria are vital organelles that are central to the energy metabolism of cells. As so-called "cellular power stations", they convert the energy contained in our food into the cell-usable energy form adenosine triphosphate (ATP). ATP is of fundamental importance for numerous cellular processes and biological functions: As a "fuel", it provides the energy required for cellular activities – for example, for cell division and many other important biochemical processes.
More than a thousand different proteins encoded in the genome of the cell nucleus are involved in this process. These so-called preproteins are synthesised in the cytosol (cytoplasm) and then imported into the mitochondria. They are bound by specific import receptors such as TOM20, TOM70 and TOM22, which deliver them to the TOM complex (translocase of the outer membrane), a complex protein machinery in the outer membrane of mitochondria. From there, they are channeled into the mitochondria and transported to their final destination by various sorting pathways. As the main entry point for protein import into the mitochondria, the TOM complex is particularly sensitive to cytosolic signalling mechanisms that can affect mitochondrial function. Under different metabolic conditions or stress, certain protein kinases can be activated that regulate the different TOM receptors through phosphorylation. Biochemist and CIBSS member Prof. Dr. Chris Meisinger and his research group at the University of Freiburg have already identified several signalling pathways for this context in earlier studies, but these were specific to individual import receptors and the associated import pathways.
The protein kinase DYRK1A as an important regulator of mitochondrial import pathways
In collaboration with Prof. Dr. Nora Vögtle, Deputy Director of the Centre for Molecular Biology at the University of Heidelberg and CIBSS member, it has now been possible to identify a signal integrator that monitors and synchronises the various import pathways ensuring that the mitochondria receive the proteins required for the respective metabolic state. This particular signalling integrator is the protein kinase DYRK1A (dual-specificity tyrosine-phosphorylation-regulated kinase 1A), which the researchers had previously identified as an activator of the TOM70 receptor. In further investigations, they were now able to show that the activity of DYRK1A regulates the quantity of the other import receptors. This leads to a remodelling of the TOM complex, enabling the various import pathways to adapt to the acute metabolic situation. "We were thus able to show for the first time that a signalling pathway in the cytosol can synchronise the various mitochondrial import pathways with each other," says Meisinger.
Scientific controversy over TOM70 phosphorylation and discovery of the special properties of DYRK1A
The researchers would not have made the discovery described in the study had they not come across the publication of another working group, which claimed that TOM70 was not phosphorylated by DYRK1A, but by a different kinase. Surprised by these contradictory results, Chris Meisinger, Nora Vögtle and their research groups comprehensively reviewed the experiments carried out there and found that an inhibitor used in the other study non-specifically inhibits the DYRK1A kinase. This activates the production of other TOM receptors, which in turn leads to the activation of TOM20-dependent import pathways, as an example. "Without the review and correction of the competing study, we would probably not have discovered the role of DYRK1A in the synchronisation of mitochondrial import pathways," says Vögtle.
The role of DYRK1A in mitochondrial dysfunction associated with Down syndrome
The protein kinase DYRK1A investigated in the Meisinger/Vögtle study plays a key role in Down syndrome. In people with Down syndrome, DYRK1A expression is increased, i.e. higher levels of the enzyme are present in the cell tissue. The researchers suspect that the activity of the mitochondrial import machinery is dysregulated as a result. This dysfunction of the mitochondrial metabolism in Down syndrome has been known for a long time; Meisinger/Vögtle's findings now provide a possible molecular explanation.
Original Publication:
Marada A., Walter C., Suhm T., Shankar S., Nandy A., Brummer T., Dhaouadi I., Vögtle F.-N., Meisinger C. (2024). DYRK1A signalling synchronizes the mitochondrial import pathways for metabolic rewiring. In: Nature Communications. 15:5265. DOI: 10.1038/s41467-024-49611-4.
CIBSS-Profile of Prof. Dr. Chris Meisinger
CIBSS-Profile of Prof. Dr. Nora Vögtle



