Known as the power plant of the cell, mitochondria are essential to human metabolism. Human mitochondria consist of 1,300 different proteins and two fatty biomembranes. The vast majority of mitochondrial proteins are produced with a cleavable transport signal and have to be actively transported into the mitochondria. Using biochemical and cell-biology experiments, a team of researchers have now shown for the first time precisely how mitochondrial proteins with signal sequences are imported into the mitochondria via a negatively charged, unique groove. Headed by Prof. Dr. Nils Wiedemann and Prof. Dr. Carola Hunte from the Medical Faculty at the University of Freiburg, and Prof. Dr. Martin van der Laan from the University of Saarland the work was carried out at the University of Freiburg’s Cluster of Excellence CIBSS – Centre for Integrative Biological Signalling Studies. Their results have been published in the science journal Nature.


Freiburg research team casts light on signal-dependent formation of mitochondria
Scientists reveal the transport of positively charged signal sequences through negatively charged groove
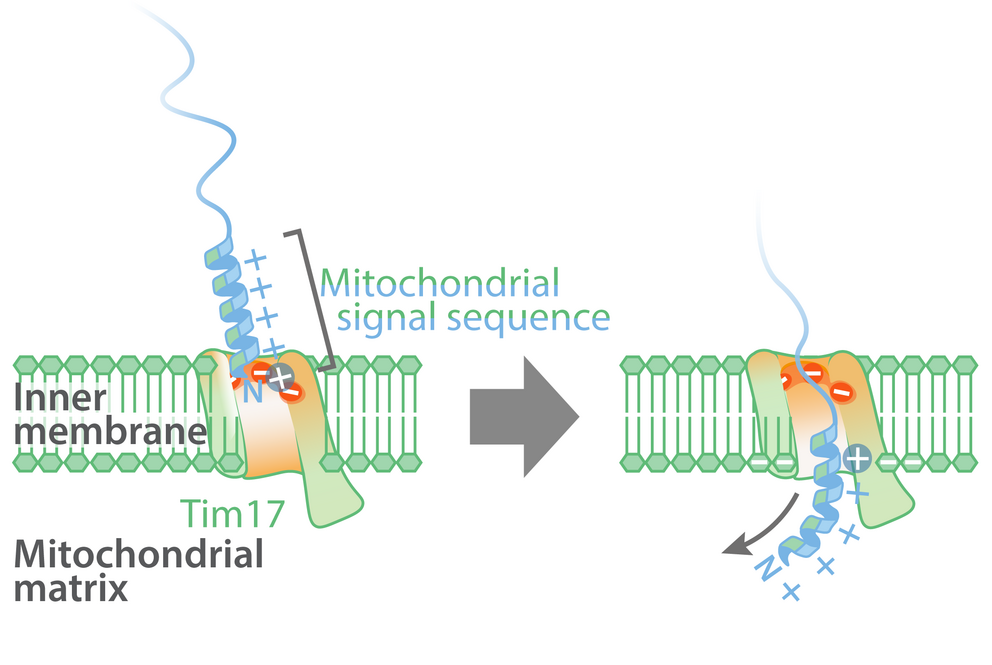
Model of the import of mitochondrial proteins with a signal sequence across the mitochondrial inner membrane at the Tim17 groove. Illustration: Laura Fielden
Transport mechanism is an important building block
“Forty years after the discovery of mitochondrial signal sequences, our experiments have now revealed the precise mechanism by which proteins are transported and the power stations of our cells are gradually built up,” says Wiedemann. “Information about the transport mechanism for mitochondrial proteins is an important component in basic cellular research.” Malfunctions of over 500 mitochondrial proteins cause a variety of diseases, so research into the mitochondria is hugely important to medicine.
It was already known that mitochondrial proteins were imported using the signal sequence translocase of the inner membrane (TIM) into the mitochondrial matrix. The two vital core subunits of this translocase are Tim17 and Tim23. Until now, it was assumed that mitochondrial proteins with signal sequences were transported across the inner membrane via a water-filled Tim23 channel. However, recent artificial intelligence-based structural predictions indicate that Tim23 does not form a channel. The research team has now been able to prove that mitochondrial proteins with signal sequences are actually imported into the mitochondria via a groove in the Tim17 protein.
Negatively charged groove in Tim17-protein
Most proteins that are transported into the mitochondria contain a complex molecular signal sequence which is positively charged and water-soluble on one side, and on the opposite side has fat-soluble molecular residues. In contrast to the positively charged side of the transport signal, the groove of Tim17 contains a strongly negatively charged region which is present in all Tim17 proteins, from yeast to humans.
The lead authors of the study, Dr. Laura Fielden and Dr. Jakob Busch from the Institute of Biochemistry and Molecular Biology at the University of Freiburg, employed functional in vitro transport experiments using chemically-marked proteins with isolated mitochondria to show that the negative charges in the groove of Tim17 interact with the positively charged signal sequences and are therefore essential to the transportation of mitochondrial proteins. Meanwhile the fat-soluble side of the mitochondrial signal sequences is aligned with the lipid membrane, enabling the transport of signal sequences at the interface between Tim17 and the mitochondrial inner membrane.
Basis for further research
“Now we have clarified this fundamental mechanism of mitochondrial proteins with a signal sequence at the interface to the biomembrane, we can understand why mitochondrial signal sequences have one positively charged side and one fat-soluble side and need this for their transportation,” Fielden explains, emphasising the importance of the results which can now serve as a basis for further research into mitochondria.
Original publication:
Fielden, L.F., Busch, J.D., Merkt, S.G., Ganesan, I., Steiert, C., Hasselblatt, H.B., Busto, J.V., Wirth, C., Zufall, N., Jungbuth, S., Noll, K., Dung, J.M., Butenko, L., von der Malsburg, K., Koch, H.-G., Hunte, C., van der Laan, M., Wiedemann, N.: Central role of Tim17 in mitochondrial presequence protein translocation. In: Nature (2023). DOI: https://doi.org/10.1038/s41586-023-06477-8



