Diploid organisms, such as humans, carry two copies of most genes: one from the biological mother and one from the biological father. One of these copies is usually silenced by epigenetic factors. The exception is haploinsufficient genes, which require two active copies. Researchers have now uncovered a control mechanism that safeguards this double-activity of specific genes: A study by the research group of Prof. Dr. Asifa Akhtar, published in the Journal Nature, identified the epigenetic regulator MSL2 as a crucial ‘anti-monoallelic’ factor that inhibits the silencing of one gene copy. This discovery also points to potential therapeutic strategies for diseases associated with haploinsufficient genes.


Revisiting gene dosage
Freiburg researchers reveal clever dosage control mechanism of biallelic genes
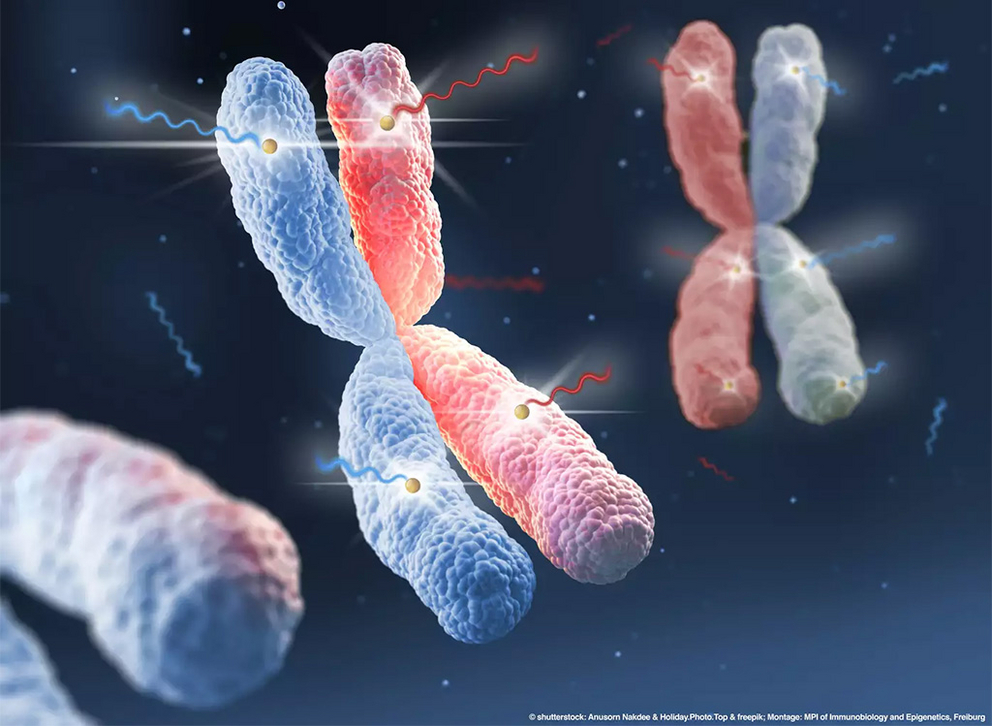
Artistic depiction of MSL2 function showing a chromosome pair (blue and red), each containing the same set of genes. The epigenetic regulator MSL2 ensures the expression of both copies of specific haploinsufficient genes (yellow dots) and guarantees that from both copies, messenger RNA is generated (zigzag lines). © shutterstock: Anusorn Nakdee & Holiday.Photo.Top; freepik, Montage: MPI-IE Freiburg
Each of the two copies of our genes, also called alleles, can serve as a template to produce messenger RNA, which in turn serves as a template for the proteins that keep our cells running. Having two alleles for each gene is thought to be the cell’s built-in redundancy system: The allele on the second chromosome can serve as a backup, which is only activated if the first one fails, for example due to a mutation. This redundancy enables diploid organisms, such as us humans, to be resistant to the effects of some mutations.
However, a class of genes known as haploinsufficient genes, rely on the continuous expression of both alleles. If just one allele of a haploinsufficient gene is compromised, it will lead to disease. Scientists therefore hypothesized that cells have a mechanism to safeguard the mRNA expression from both alleles for this special class of genes. The current study led by Akhtar describes exactly such a mechanism.
MSL2 is an epigenetic dosage-sensor
With MSL2, the research team has identified, for the first time, a protein that can sense dosage-sensitive genes and ensure their biallelic expression in the relevant tissue or during certain developmental stages.
“We were always wondering whether the copy of the gene on the chromosome coming from the mother could communicate with the second copy on the chromosome coming from the father. Our findings imply an underlying communication between the two alleles and we speculate that MSL2 ensures that mom and dad can talk to each other – at least molecularly,” says Akhtar, Director at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg and member of the Cluster of Excellence CIBSS – Centre for Integrative Biological Signalling Studies at the University of Freiburg.
Tracking down the allelic regulator with a genetic trick
Fascinated by their discovery, the researchers investigated how this mechanism works at the molecular level. To tackle this, the team used a trick. "We crossed genetically distant mouse strains with each other - a bit like crossing a Chihuahua with a Great Dane. This allowed us to see which alleles were inherited from the mother and which from the father," says Yidan Sun, the first author of the paper, explaining the method of allele-specific gene expression analysis. With this hybrid mouse system, the team could analyze the activity of individual alleles. She adds: "In contrast to the standard method of expression data analysis, in which the gene products are summed over the two alleles, this gave us the resolution necessary to track the expression status of each allele individually".
A future for novel therapeutic strategies to address diseases
Their experiments demonstrated that when MSL2 was lost in hybrid mouse cells, only one copy of certain haploinsufficient genes was still expressed. This implies that in mammalian cells, MSL2 is necessary for the biallelic expression of genes, ensuring their functionality and, consequently, the overall health of the organism. Interestingly, many of the haploinsufficient genes regulated by MSL2 are associated with neurological disorders.
“But what adds a fascinating layer to this discovery is the tissue- and cell-type specificity of these genes. Looking at the organism as a whole, it makes you wonder whether a backup system orchestrated by epigenetic factors such as MSL2 might explain why people, even with similar lifelong habits like smoking or diet, have different health outcomes or disease risks," says Meike Wiese, one of the first authors of the study.
An evolutionarily conserved mechanism
“My lab started out studying dosage compensation in fruit flies, which is the process by which males with one X chromosome can achieve the same level of gene products as females with two X chromosomes. Over the years we have been fascinated by how male fruit flies with just one X chromosome do double duty to produce the same messenger RNA compared to the females with two X chromosomes. Without this double dose males simply die! It looks like this strategy has been cleverly adapted by mammals. Our results clearly illustrate how the same tools, like MSL2, are again used in evolution to regulate dosage of genes. Gene dosage matters, and our study provides a new level of understanding of how the cells in our body ensure that we get the right dose of messenger RNAs,” says Akhtar.
What excites the scientists is that this discovery opens new directions to delve deeper into understanding the modulation of gene dosage within our cells. MSL2, as revealed, may just be one example of such an allelic regulator, suggesting the existence of other factors performing similar roles. This newfound knowledge carries profound implications for understanding diseases and holds promise for developing potential treatments.
Original publication
Sun Y, Wiese M, Hmadi R, Karayol R, Seyfferth J, Martinez Greene JA, Erdogdu NU, Deboutte W, Arrigoni L, Holz H, Renschler G, Hirsch N, Foertsch A, Basilicata MF, Stehle T, Shvedunova M, Bella C, Pessoa Rodrigues C, Schwalb B, Cramer P, Manke T, Akhtar A (2023). MSL2 ensures biallelic gene expression in mammals. In: Nature. DOI: 10.1038/s41586-023-06781-3



