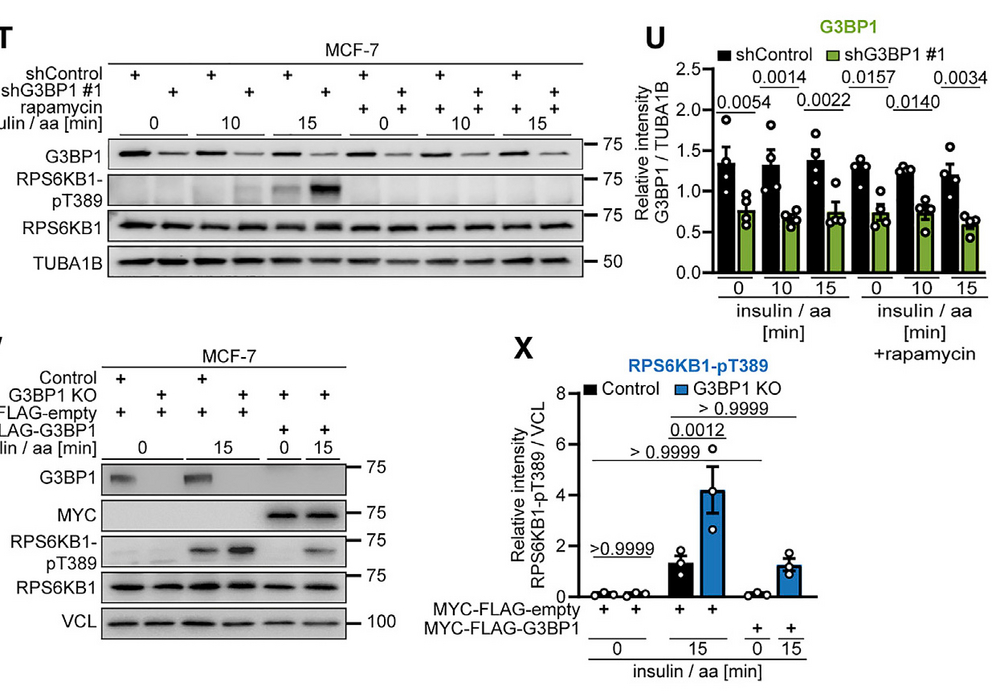
The G3BP (Ras GTPase-activating protein-binding protein 1 and 2) proteins are widely recognized as core components of stress granules (SG). We report that in cells without SG, the G3BP proteins reside at the cytoplasmic surface of lysosomes. They act in a non-redundant manner to anchor the Tuberous Sclerosis Complex (TSC) protein complex to lysosomes and inhibit the metabolic master regulator mTORC1 (mechanistic target of rapamycin complex 1). Like the TSC complex, G3BP1 deficiency elicits phenotypes related to mTORC1 hyperactivity in the context of tumors and neuronal dysfunction. Thus, the G3BP proteins are not only core components of SG but also a key element of lysosomal TSC-mTORC1 signaling.


G3BPs tether the TSC complex to lysosomes and suppress mTORC1 signaling