Dr. Dr. Bertram Bengsch (CIBSS-PI), Department of Internal Medicine II (University Medical Center Freiburg, Faculty of Medicine)
T cells are key to the ability of the immune system to control infection, cancer and prevention of autoimmunity. However, in chronic viral infection or cancer, T cell functionality is altered and insufficient to prevent pathology. We are interested in how the T cell response is regulated and exhausted during chronic inflammation and how immunotherapeutic interventions can augment function.
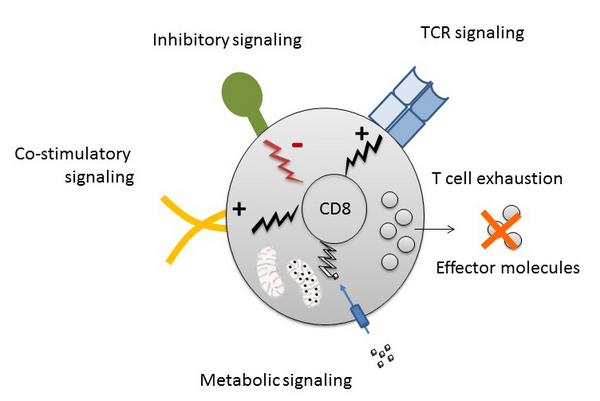
A major focus in the lab is centered on understanding exhausted T cells (TEX), which constitute a T cell lineage distinct from functional memory and effector cells that induced during chronic infection, cancer and autoimmune disease. TEX are characterized by co-expression of immunoregulatory molecules, an altered transcriptional and epigenetic landscape and reduced effector and memory functionality. TEX co-integrate signals from the T cell receptor and immune checkpoints (e.g., the inhibitory receptors PD-1, CTLA-4). We have recently demonstrated heterogeneity and disease associations of different varieties of exhausted T cells in humans that are impacted by antiviral and checkpoint therapy. Mechanistically, we identified the control of T cell metabolism as a hallmark of T cell exhaustion that occurs downstream of inhibitory receptor signalling. However, the precise mechanisms how metabolism is regulated downstream of TCR and co-regulatory signalling and how this influences T cell differentiation remain unclear. In this project, we will investigate how modulation of cellular and mitochondrial metabolism affects exhausted human T cells. We recently defined novel metrics to precisely characterize human TEX in chronic infection and cancer. We will focus on our recent advances in the understanding of T cell exhaustion to test and manipulate metabolic and signalling pathways that are specifically regulated in exhausted cells and contribute to the altered (dys)function of these T cells. We will use high-parametric flow cytometry, including advanced exhaustion-directed high-dimensional profiling approaches, phosphoflow, metabolic flux profiling and cell culture assays. In later stages of the project, mass cytometry and single-cell transcriptomics, epigenomics and metabolomics are envisioned.
In sum, this project is expected to provide insights into the intersection of metabolic signalling and T cell signalling and highlight pathways amenable to intervention. Understanding the regulation of exhausted T cells has major implications for immunotherapeutic approaches in infection, cancer and autoimmunity.