Jun. Prof. Dr. Katrin Kierdorf (CIBSS-AI), Institute of Neuropathology (University Medical Center Freiburg, Faculty of Medicine)
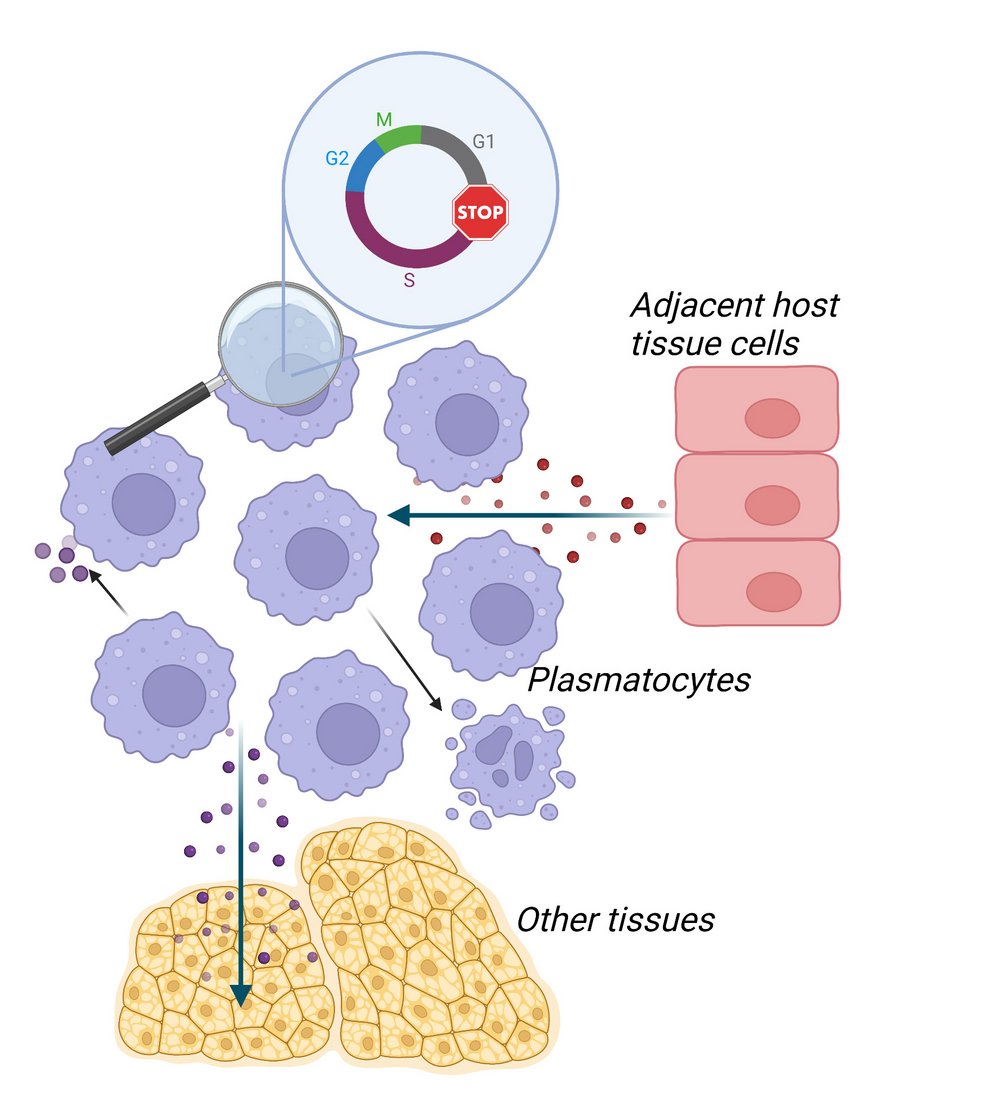
Tissue resident macrophages are essential contributors to tissue homeostasis as well as immune defence in various organs. Therefore, the control of their cell number within the tissue niche is detrimental for physiological function of the organs and an adequate immune response. Plasmatocytes, the macrophage-like immune cells of Drosophila melanogaster, originate from embryonic and larval hematopoiesis and share conserved functions and signalling pathways with vertebrate macrophages. During embryonic, larval and pupal stages, plasmatocytes proliferate and expand throughout the tissues. However as soon as the imago ecloses from the pupal case, plasmatocytes rapidly lose their proliferation capacity and coordinate a tightly regulated cell cycle arrest. Interestingly even immune challenges or depletion of plasmatocytes in adult Drosophila do not facilitate cell cycle re-entry. We and others have previously demonstrated that alterations in plasmatocyte numbers can have detrimental effects on tissue homeostasis. Though it remains ill-defined which extrinsic and intrinsic signals regulate the cell cycle exit and define macrophage cell number in adult Drosophila. In the proposed project we will decipher signals in control of plasmatocyte cell number in adult Drosophila. We will focus on the definition of the signalling network inducing the coordinated cell cycle arrest of plasmatocytes in newly eclosed adult Drosophila. Advances in understanding the signalling machinery controlling immune cell number in Drosophila would further allow us to identify potential conserved signals from invertebrates to mammals.