Dr. Peter Walentek (CIBSS-AI), Internal Medicine IV, University Medical Center Freiburg
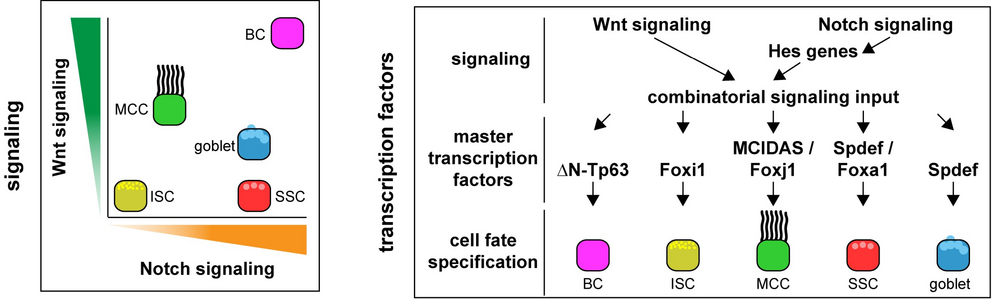
Elucidating signalling functions and integration is pivotal to understand the principles of self-organization inherent to developing systems. In vertebrates, mucociliary epithelia can line the epidermis and several inner organs, e.g. the airways. They are comprised of a set of specialized cell types, including basal stem cells, multiciliated cells and secretory cell types. Collectively, they provide a first-line of defence against pathogens to the organism through mucociliary clearance. In our studies addressing the signalling contributions to self-organized pattern formation, we have already demonstrated how Wnt signalling regulates basal stem cells and ciliation (Haas et al. 2019), and how Notch signalling regulates mucociliary tissue remodelling in development (Tasca et al. 2021). In our previous CIBSS project (B14), we have uncovered how a Notch-dependent signalling algorithm regulates cell fate specification through temporal signalling integration. Our preliminary data as well as emerging literature indicate an important contribution of epigenetic control mechanisms to mucociliary development and disease. Therefore, we will continue our investigations of mucociliary signalling, but now focus on elucidating how spatiotemporal signalling input is integrated at the genomic level by epigenetic regulatory mechanisms. We will elucidate how cellular systems employ dynamic signalling to communicate and understand each other, a prerequisite for self-organization phenomena in biology, and investigate new paradigms in both spatial and temporal signalling integration at the chromatin level.