Prof. Dr. Dr. Bertram Bengsch (CIBSS-PI), Department of Internal Medicine II - Gastroenterology, Hepatology, Endocrinology and Infectionology, University Medical Center, Faculty of Medicine
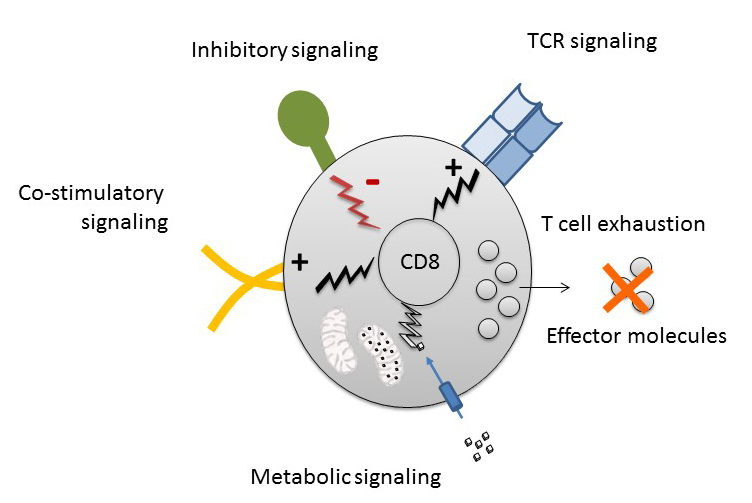
T cell exhaustion arises in the context of chronic viral infections, cancer and autoimmunity. Exhausted T cells (TEX) experience persistent T cell receptor (TCR) stimulation while encountering inhibitory signals through receptors such as PD-1. Inhibitory receptor blockade targeting TEX is key to the success of immunotherapeutic approaches. We previously identified the control of T cell bioenergetics due to PD-1 signalling as a hallmark of TEX that involves changes in TCR-driven mTOR signalling, control of glycolysis and mitochondrial function with elevated ROS production [1]. We also identified significant heterogeneity within exhausted T cells and defined precursor populations with memory-like features [2, 3] and different metabolic properties. However, how the key signals, TCR signalling, co-stimulation/inhibition and metabolic cues integrate to drive the program of exhaustion and if that determines different subsets remains unclear. In this project we will perform a highly multiplexed phospho-signalling CyTOF analysis in Jurkat leukemia cell lines and primary human T cells from patients with conditions driving T cell exhaustion, such as chronic infection (e.g., Hepatitis B or C Virus infection), cancer (melanoma, hepatocellular carcinoma) and healthy individuals to determine the TCR and metabolic downstream signalling in exhausted and canonical human T cells. We will then use pharmacologic and genetic gain- and loss of function approaches to interfere with different mitochondrial functions to assess the effect on features of exhaustion. Lastly, we will determine how altered integration of TCR signalling (Signal 1), Co-stimulatory/Co-inhibitory signalling (Signal 2) and metabolic signalling (Signal 3) impacts on T cell function and differentiation of exhausted T cells by optogenetic control-of-function. This project is based on collaborations with the groups of M. Hoerner, G. Radziwill and W. Schamel.