Prof. Dr. Michael Koettgen (CIBSS-AI), Zentrale Klinische Forschung (University Medical Center Freiburg, Faculty of Medicine)
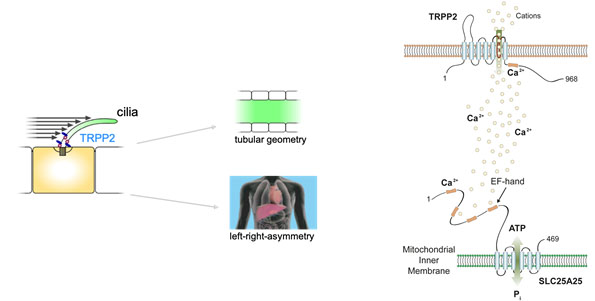
Mutations in transient receptor potential channel polycystin-2 (TRPP2) cause autosomal dominant polycystic kidney disease (ADPKD) and left-right patterning defects. TRPP2 is a Ca2+-permeable cation channel, but it is not known how TRPP2 channel activity translates into morphogenetic programs controlling the shape of epithelial tubules and left-right patterning in vertebrates. We hypothesize that local Ca2+ signals are critical to regulate specific downstream factors in TRPP2 signalling micro-domains to control cell and organ shape. We have identified novel proteins in the TRPP2 signalling cascade using a combination of unbiased proteomic and forward genetic approaches. These studies have uncovered a novel mechanistic link between cilia-mediated Ca2+ signalling and mitochondrial metabolism as well as actin-myosin-mediated cell shape regulation. The spatiotemporal control and integration of these signalling events is unknown and shall be studied in this project. We will analyse the spatiotemporal regulation of calcium and metabolic signals using genetically encoded sensors (GCAMP6 and ATeam). Furthermore, we will apply control of function approaches using Channelrhodopsin 2 to determine the spatiotemporal requirements of metabolic signalling in polarized epithelial cells. To investigate signal integration, we will study the functional hierarchy of the molecular players in cell culture and zebrafish. Our project will address the mechanisms of TRPP2 signalling over a wide range of spatiotemporal scales: from Ca2+ signals operating in seconds to organ development and maintenance ranging from days to life time. We anticipate that this project will provide insights into the fundamental mechanisms of TRPP2-mediated signal transduction, providing a potential entry point into designing targeted therapeutic approaches for ADPKD.