Prof. Dr. Chris Meisinger (CIBSS-PI), Institute of Biochemistry and Molecular Biology (Faculty of Medicine), University of Freiburg
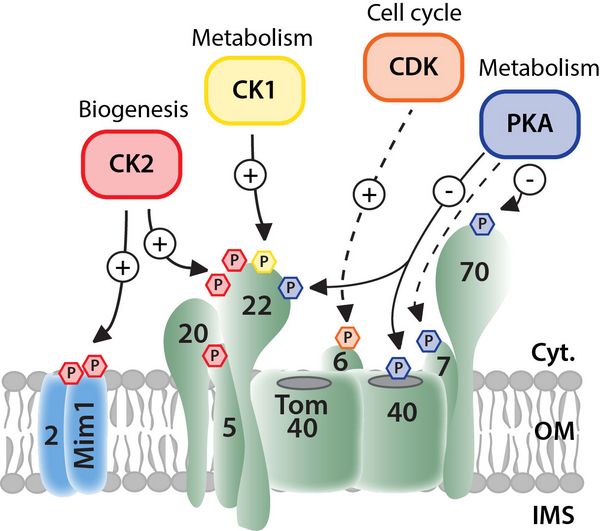
The almost entire mitochondrial proteome, which comprises approx. 1500 different proteins in humans, is build up by the import of nuclear encoded precursor proteins from the cytosol. This process is mediated by several sophisticated protein machineries in the outer and inner membrane that translocate and - dependent on the presence of a certain type of signal sequence within the precursors - sort the preproteins to their respective suborganellar destination. The mitochondrial protein import machinery has been considered to be constitutively active and work in a rather static manner. However, our recent studies in the model organism Saccharomyces cerevisiae have revealed that the import machinery is the target of several cytosolic and mitochondrial protein kinases implying a tight connection of mitochondrial protein biogenesis and cellular signaling cascades. Particularly, the translocase of the outer membrane (TOM complex) was found modulated by protein kinases that target more than 30 different phosphorylation sites at the various TOM proteins. This allows a dynamic regulation of TOM biogenesis and function, which is important to adapt the organellar proteome to changing cellular demands upon metabolic switches or to link protein import activity to certain stages of the cell cycle. We will analyze signalling mechanisms across different cell cycle phases and across different cellular compartments, from nucleus, cytosol to mitochondria, to understand how mitochondrial function(s) can be adapted to changing cellular demands during the different phases of cell growth and division.