Dr. Edward Pearce (CIBSS-AI) and Dr. Erika Pearce, Max Planck Institute of Immunobiology and Epigenetics Freiburg
CIBSS Profile page Eward Pearce
CIBSS Profile page Erika Pearce
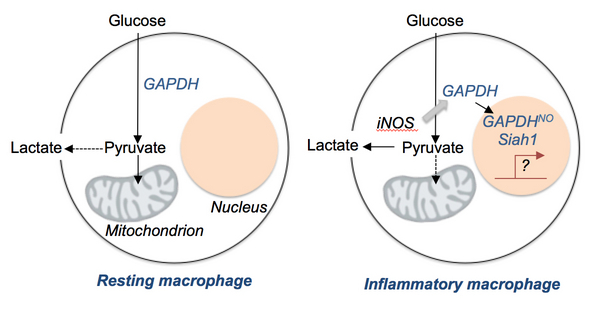
Activation in immune cells is associated with metabolic remodelling which includes increased glycolysis (O’Neill and Pearce, J Exp Med, 2016; Buck et al. Cell, 2017). This pathway is critical since in many cases its inhibition results in diminished activation. Despite many possibilities, the actual reason why glycolysis is critical for activation is not clear. This question is particularly interesting in inflammatory macrophages, in which high levels of nitric oxide (NO) are produced following the expression of inducible NO synthase. GAPDH, a rate limiting and central enzyme in the glycolysis pathway, can be nitrosylated by NO, and in this form is enzymatically inactivated but able to interact with other proteins (including the E3 ubiquitin ligase Siah1) and perform distinct non-glycolysis related transnitrosylation functions within the nucleus that can affect gene expression (Kornberg et al. Nat Cell Biol, 2010). The impact of these events in inflammatory macrophages is unclear, and is the subject of this proposal. In our project we will assess the role of NO made by inducible NO synthase (iNOS) on GAPDH localization and function (collab. EL Pearce), and activation-induced epigenetic and transcriptional changes in gene expression in inflammatory macrophages (collab. A Akhtar), and we will explore the role of Siah1 in metabolic reprogramming, GAPDH localization, and activation in macrophages exposed to inflammatory stimuli (collab. M Prinz). This area of research has strong translational potential since manipulating metabolism to alter immune cell function has promise to alter the course of autoimmune diseases and cancer.