Dr. Nora Vögtle (CIBSS-AI), Institute of Biochemistry and Molecular Biology (Faculty of Medicine), University of Freiburg
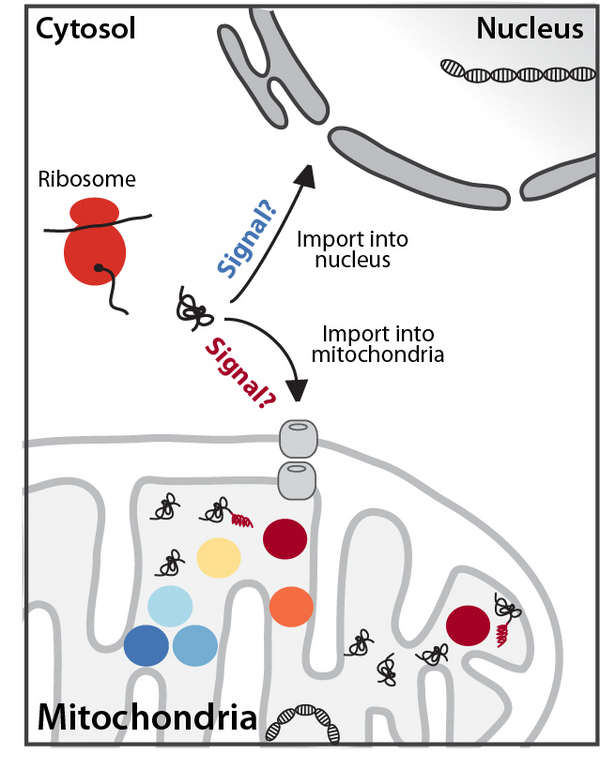
Mitochondrial proteostasis and quality control are essential for cell survival and mitochondrial dysfunction results in mito-nuclear communication termed mtUPR (mitochondrial unfolded protein response) pathways. We have identified several mutations in patients that result in imbalanced mitochondrial protein biogenesis and that lead to severe disorders such as cardiomyopathy or neurodegeneration. By applying a temperature-sensitive mutant strain we were able to induce a reversible mitochondrial stress pathway that is unique as it enables the investigation and dissection of the early steps in mtUPR. Cell survival during early stress induction depends on a nuclear transcription factor sTFA that retranslocates to mitochondria upon short term mtUPR induction, where it binds mtDNA and maintains mitochondrial transcription. Loss of sTFA results in dysfunctional mitochondrial metabolism and ultimately cell death.
We will address how defects in mitochondrial proteostasis are transduced to the nucleus and which signaling cascades are activated to rescue mitochondrial dysfunction and ultimately cell survival. This knowledge will be exploited to understand the course of disease in patients with mutations affecting mitochondrial protein biogenesis and ultimately open new avenues for therapeutic interventions.
Publications resulting from the project
Increased mitochondrial protein import and cardiolipin remodelling upon early mtUPR.
Poveda-Huertes D, Taskin AA, Dhaouadi I, Myketin L, Marada A, Habernig L, Büttner S, Vögtle FN
PLoS Genet 2021; doi: 10.1371/journal.pgen.1009664.
An Early mtUPR: Redistribution of the Nuclear Transcription Factor Rox1 to Mitochondria Protects against Intramitochondrial Proteotoxic Aggregates.
Poveda-Huertes D, Matic S, Marada A, Habernig L, Licheva M, Myketin L, Gilsbach R, Tosal-Castano S, Papinski D, Mulica P, Kretz O, Kücükköse C, Taskin AA, Hein L, Kraft C, Büttner S, Meisinger C, Vögtle FN
Mol Cell 2020; doi: 10.1016/j.molcel.2019.09.026.