Deciphering TIM-3 signaling to enhance anti-leukemia immunity against AML
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is the primary curative treatment for patients with acute myeloid leukemia (AML). The therapeutic benefit of allo-HCT relies on a robust T cell-mediated graft-versus-leukemia effect (GVL)1. However, allo-HCT as treatment for AML has two major limitations: (i) AML tumor relapse, still occurring in around 40% of patients2 and (ii) the development of graft-versus-host disease (GVHD). GVHD represents the most common life-threatening complication where donor T cells recognize, target and attack healthy tissues in the recipient3,4. While relapse after allo-HCT remains a major therapeutic challenge, several immune escape mechanisms involved have been identified, including the overexpression of inhibitory receptor ligands by AML cells, leading to direct inhibition of immune cell function.
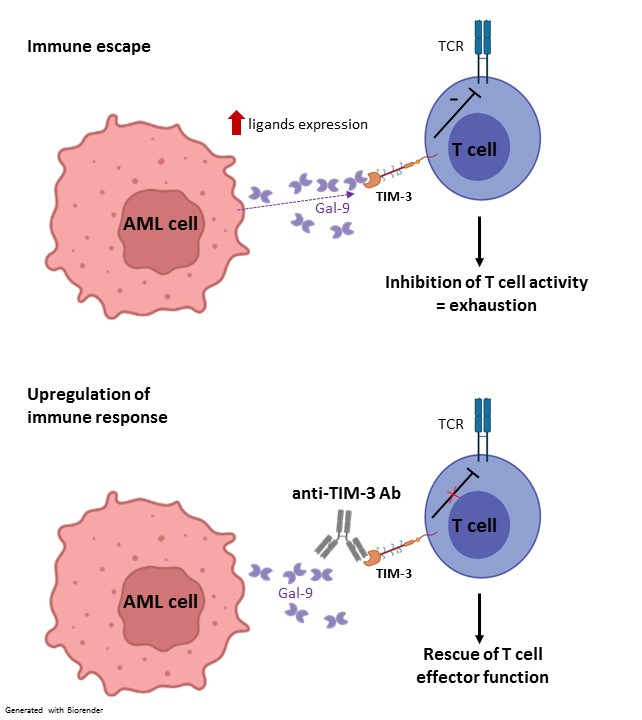
TIM-3, first identified as a molecule expressed on interferon-γ producing T cells, has recently emerged as an inhibitory receptor (IR)5,6, and more broadly, as a marker for T cell dysfunction/exhaustion in cancer7, which makes it a promising target for enhancing T cell immunity against AML. Exhaustion is a functional state characterized by a loss of proliferative capacity, decreased cytokine production and reduced cytotoxic activity5. In addition, TIM-3 and its ligand Galectin-9 are highly expressed on leukemic stem cells, inducing an autocrine loop that is essential for their self-renewal and AML development8.
In this project, I aim to investigate the mechanisms of TIM-3 blockade to overcome immune escape and reinvigorate immune cells to enhance anti-leukemia immunity after allo-HCT in AML.
Aim 1 To study TIM-3 blockade in the allogenic context, including signaling pathway networks (still not fully understood), metabolic status of donor cells or with regard to GVHD development.
Aim 2 To decipher the interaction between oncogene signaling and IR(s) / ligand(s) expression to identify intrinsic resistance or beneficial mutations to TIM-3 blockade, leading to personalized treatment(s) and/or prognostic biomarker(s).
Aim 3 To characterize ICI combinations to overcome potential treatment resistance.
Immune-checkpoint inhibitors monotherapies often lead to the acquisition of Ab resistance (e.g., aberrant cell signaling), inducing tumor relapse and immune-related adverse events (irAEs), resulting in inflammation9. Combination treatment with immunotherapies appears to be a favorable strategy to prolong the activation of the immune response, decrease immunosuppression, and target signaling and resistance pathways, leading to a more durable and long-lasting treatment.
References
1. M. M. Horowitz et al. Blood 75, 555-562 (1990).
2. P. Tsirigotis et al. Bone Marrow Transplant 51, 1431-1438 (2016).
3. G. Socie, J. Ritz. Blood 124, 374-384 (2014).
4. R. Zeiser, B. R. Blazar. N Engl J Med 377, 2167-2179 (2017).
5. E. J. Wherry, M. Kurachi. Nat Rev Immunol 15, 486-499 (2015).
6. K. O. Dixon et al. Nature 595, 101-106 (2021).
7. M. Hashimoto et al. Annu Rev Med 69, 301-318 (2018).
8. Y. Kikushige et al. Cell Stem Cell 17, 341-352 (2015).
9. K. L. Reynolds et al. J Immunother Cancer 9, (2021).