In vivo Identification of Kinase Phosphorylation Targets Using Analog-Sensitive Kinases and Next-Generation ATP Probes
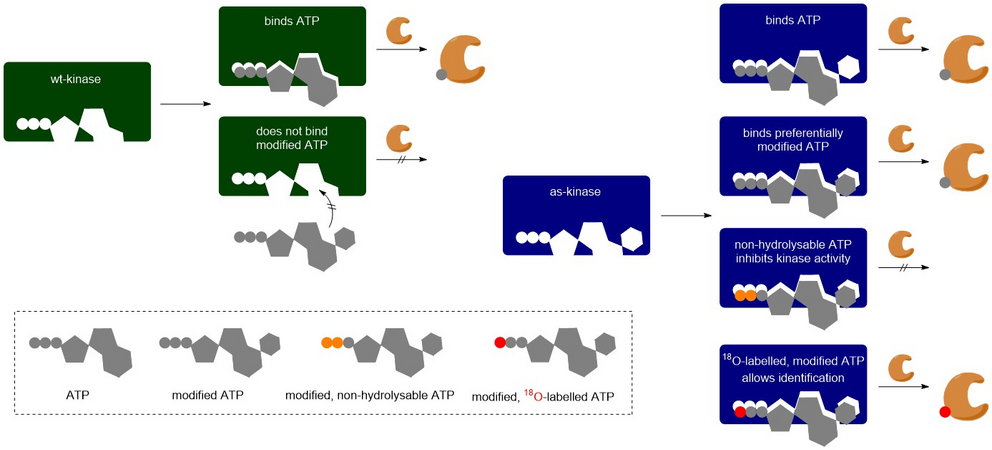
Reversible phosphorylation is a key regulatory mechanism, controlling processes such as growth, differentiation, and stress responses. Despite its importance, identifying direct kinase-substrate relationships and understanding the timing of phosphorylation events in vivo remain major challenges. A central limitation is that all kinases rely on the same intracellular ATP pool, making it difficult to distinguish the activity of individual kinases under physiological conditions. Traditional approaches, such as radioactive labelling or thiophosphate analogues, are mostly restricted to in vitro use and often lack temporal resolution or disrupt cellular integrity.
Our project addresses these limitations by developing a novel system based on light-activated, cell-permeable ATP analogues labelled with heavy oxygen isotopes. These analogues are selectively used by engineered analog-sensitive (as-) kinase variants, allowing specific and temporally controlled labelling of its substrates in vivo. Combined with high-resolution phosphoproteomics, this approach will enable precise mapping of kinase targets and their phosphorylation kinetics, establishing a broadly applicable platform for dissecting signalling networks in living cells. The first step will now be to synthesize a variety of ATP analogs to determine optimal substrates for certain as-kinases.