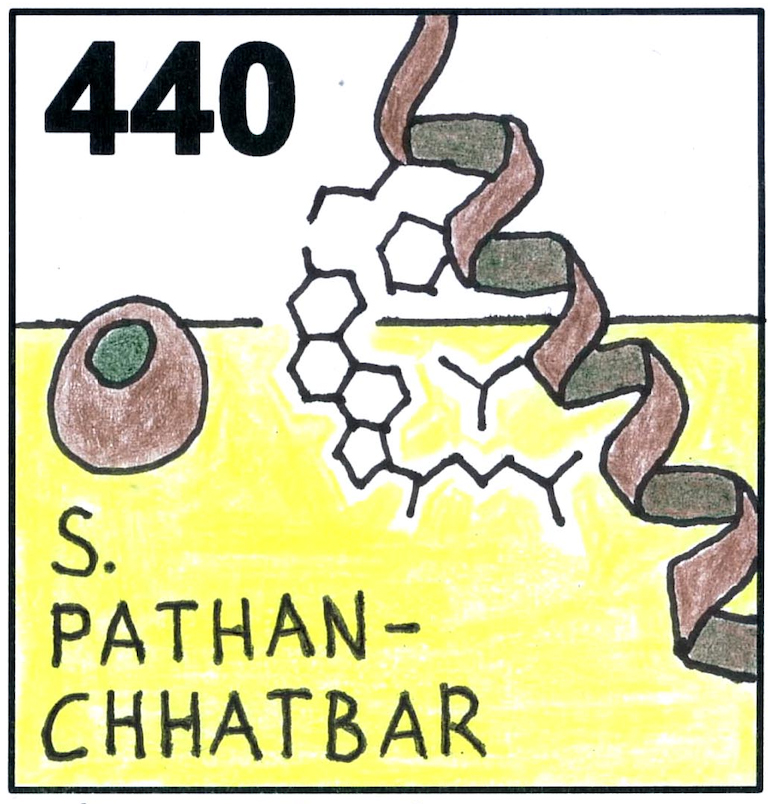
Earlier we have found that the membrane lipid cholesterol binds to the transmembrane (TM) region of the T cell receptor beta (TCRbeta) chain and thereby regulates the signalling activity of the TCR. Together with our cooperation partner from Shanghai, China, we have now solved the structure of cholesterol binding to the isolated TCRbeta TM region. Using NMR and molecular dynamics simulations, we found that cholesterol binds to the TM residues within a CARC-like cholesterol recognition motif. Surprisingly, the polar OH group of cholesterol is placed in the hydrophobic center of the lipid bilayer stabilized by its polar interaction with lysine 154 of the TCRbeta TM region. An aromatic interaction with tyrosine158 and hydrophobic interactions with valine 160 and leucine 161 stabilize this reverse orientation.


Cholesterol binds in a reversed orientation to TCRβ-TM in which its OH group is localized to the center of the lipid bilayer